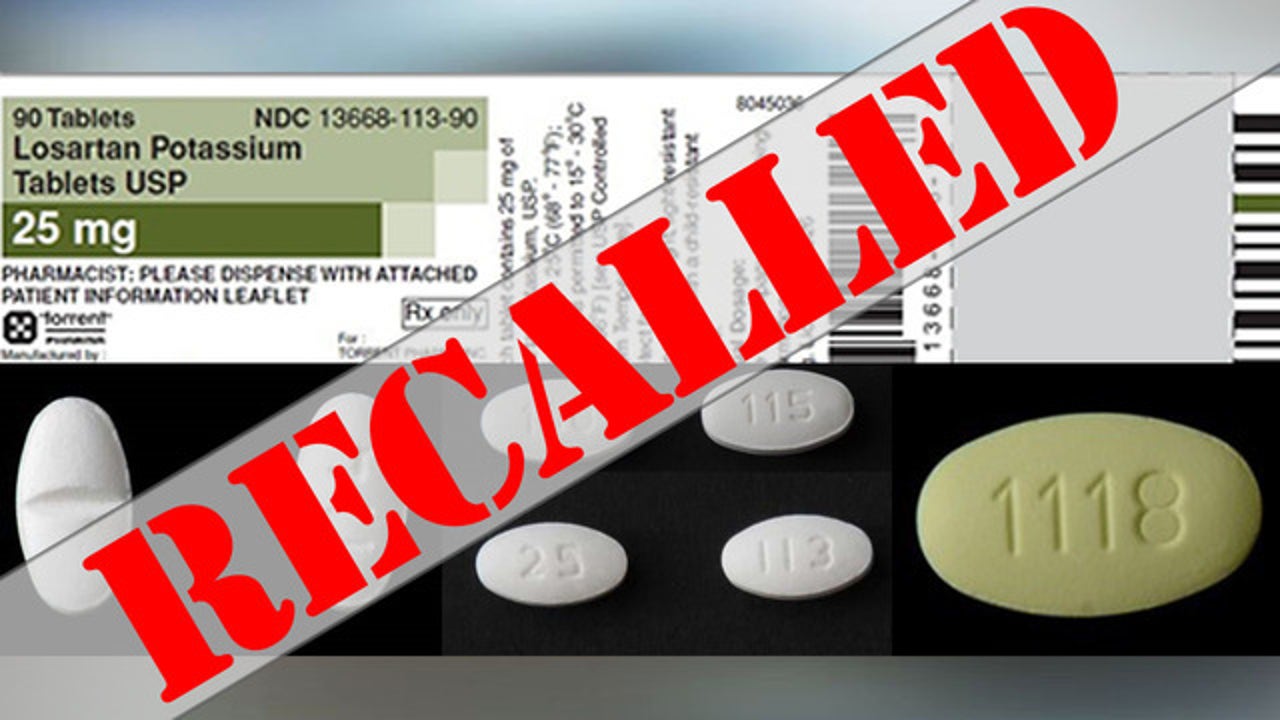
Losartan 100mg Recall 2024 – A total of 1513 patients were enrolled in this randomized, double-blind study comparing losartan (50 to 100 mg once daily) with placebo, both taken in addition to conventional antihypertensive . The product was discontinued in September 2023. It comes after the US Food and Drug Administration issued two recalls – one on December 15 and another on January 11 – affecting more than 100 products. .
Losartan 100mg Recall 2024
Source : trumedsystems.comBlood pressure medication recalled after possible cancer causing
Source : www.fox5atlanta.comFDA: Blood pressure medication recall expanded again for risk of
Source : www.mytwintiers.comBlood pressure medication recall expanded after possible cancer
Source : www.fox5atlanta.comBlood pressure medication recalled due to ‘unexpected impurity’
Source : www.wbtv.comBlood pressure medication recall expanded after possible cancer
Source : www.fox5atlanta.comRecall of Blood Pressure Drug Losartan Expanded
Source : mynews13.comRECALL: Losartan recalled again eParisExtra.com
Source : eparisextra.comBlood pressure medication recall expanded after possible cancer
Source : www.fox5atlanta.comThe FDA again adds more drugs to its valsartan recall list | CNN
Source : www.cnn.comLosartan 100mg Recall 2024 How to Track and Manage Recalled Drugs TruMed Systems: More than 100 products are under recall because of listeria. You can find the full list of recalled items here. It could cause serious illness, and many of the affected items are sold in Ohio. . Decades after her service in the Women’s Army Corps, there’s no doubt that Emma Pogge emerged relatively unscathed from her experiences in post-war Germany. Considering the world climate at .
]]>